Dosing & MOA
Available in Two Convenient Strengths and Formulations1
Two strengths
250 mg
or
500 mg
Two dosing formulations
capsules
or
powder for oral suspension
Both formulations can be stored at room temperature (68°F – 77°F).
DIACOMIT should be taken during a meal:
- DIACOMIT capsules must be swallowed whole with a glass of water
- DIACOMIT powder for oral suspension should be mixed in a glass of water (100 mL) and should be taken immediately after mixing
Recommended Oral Dosage of 50 mg/kg/day per US Prescribing Information1
The total maximum dosage is 3,000 mg/day. In the event of a missed dose, DIACOMIT should be taken as soon as possible. If it is almost time for the next dose, the patient should not take the missed dose. Doses should not be doubled.1
| 6 MONTHS TO 1 YEAR*† | 1 YEAR AND OLDER | ||
|---|---|---|---|
| BODY WEIGHT | ≥ 7kg | 7kg to < 10kg† | ≥ 10kg |
| DOSE | 50mg/kg/day
Divide the total daily dose into 2 doses |
50mg/kg/day
Divide the total daily dose into 2 doses |
50mg/kg/day
Divide the total daily dose into 2 doses |
|
Round to the nearest possible dosage, not to exceed 3,000 mg/day |
|||
| 6 MONTHS TO 1 YEAR*† | |||
|---|---|---|---|
| BODY WEIGHT | ≥ 7kg | ||
| DOSE | 50mg/kg/day
Divide the total daily dose into 2 doses |
||
|
Round to the nearest possible dosage, not to exceed 3,000 mg/day |
|||
| 1 YEAR AND OLDER | |||
| BODY WEIGHT | 7kg to < 10kg† | ||
| DOSE | 50mg/kg/day
Divide the total daily dose into 2 doses |
||
|
Round to the nearest possible dosage, not to exceed 3,000 mg/day |
|||
| 1 YEAR AND OLDER | |||
| BODY WEIGHT | ≥ 10kg | ||
| DOSE | 50mg/kg/day
Divide the total daily dose into 2 doses |
||
|
Round to the nearest possible dosage, not to exceed 3,000 mg/day |
|||
*Dosing frequency should not exceed twice daily to limit free water administration.
†Dosing frequency should not exceed twice daily to avoid overexposures.
European Union Label and Real-World Dosing Recommendations2,3
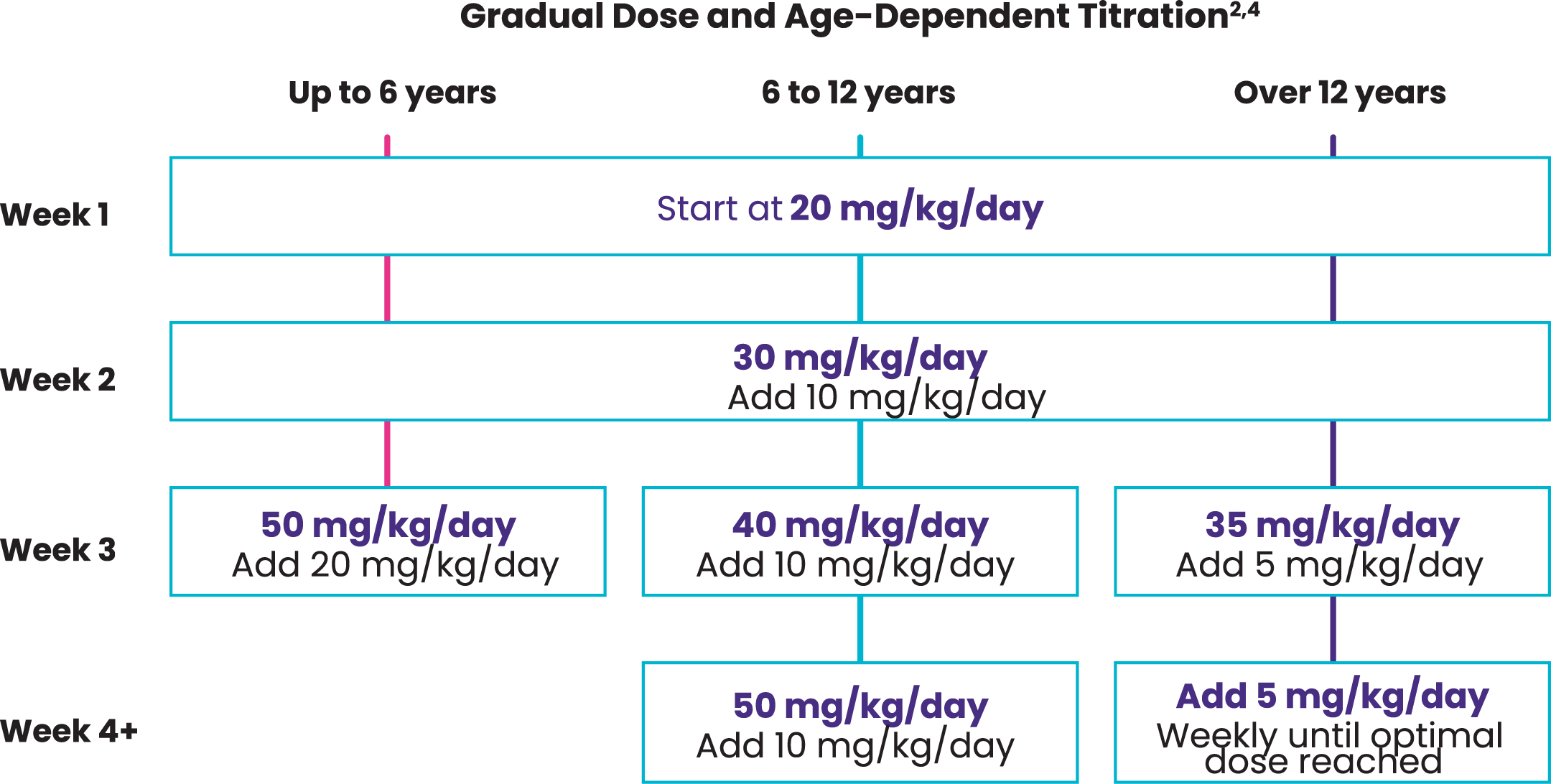
| Up to 6 years | |
|---|---|
| Week 1 | Start at 20 mg/kg/day |
| Week 2 | 30 mg/kg/day Add 10 mg/kg/day |
| Week 3 | 50 mg/kg/day Add 20 mg/kg/day |
| 6 to 12 years | |
| Week 1 | Start at 20 mg/kg/day |
| Week 2 | 30 mg/kg/day Add 10 mg/kg/day |
| Week 3 | 50 mg/kg/day Add 20 mg/kg/day |
| Week 4+ | 50 mg/kg/day Add 20 mg/kg/day |
| Over 12 years | |
| Week 1 | Start at 20 mg/kg/day |
| Week 2 | 30 mg/kg/day Add 10 mg/kg/day |
| Week 3 | 50 mg/kg/day Add 20 mg/kg/day |
| Week 4+ | 50 mg/kg/day Add 20 mg/kg/day |
Start Low, Go Slow5
- A “start low, go slow” titration approach is often employed to reduce the risk of serious adverse effects and tolerability issues, allowing patients to safely reach an optimal maintenance dose5
- A population pharmacokinetic model of stiripentol in children with Dravet, who were also taking clobazam and valproate, confirmed the dose-dependent non-linearity observed in adults4
DIACOMIT Has No Known Contraindications*1
While DIACOMIT has no known contraindications, dose adjustments to DIACOMIT and other drugs may be needed to mitigate potential interactions.
| ASM | Interaction | Recommendation |
|---|---|---|
| Clobazam1 | 2-to-3-fold increase in clobazam and 5-fold increase in norclobazam plasma concentrations | Reduce CLB by 25% If somnolence persists, further reduce by an additional 25% and adjust dosage of other concomitant anticonvulsant drugs with sedating properties |
| Valproate1,6 | The potential for metabolic interaction is considered modest | Reduce VPA by 30% or 10 mg/kg daily in case of loss of appetite |
| Cannabidiol7 | No change in CBD levels and clinically insignificant increases in 7-OH-CBD | N/A |
| Fenfluramine8 | Increase in fenfluramine plasma concentrations when coadministered with stiripentol and clobazam | When coadministered with stiripentol and clobazam, maximum fenfluramine dose is 0.2 mg/kg twice daily (max 17 mg/day) |
| Clobazam1 |
|---|
| Interaction |
| 2-to-3-fold increase in clobazam and 5-fold increase in norclobazam plasma concentrations |
| Recommendation |
| Reduce CLB by 25% If somnolence persists, further reduce by an additional 25% and adjust dosage of other concomitant anticonvulsant drugs with sedating properties |
| Valproate1,6 |
| Interaction |
| The potential for metabolic interaction is considered modest |
| Recommendation |
| Reduce VPA by 30% or 10 mg/kg daily in case of loss of appetite |
| Cannabidiol7 |
| Interaction |
| No change in CBD levels and clinically insignificant increases in 7-OH-CBD |
| Recommendation |
| N/A |
| Fenfluramine8 |
| Interaction |
| Increase in fenfluramine plasma concentrations when coadministered with stiripentol and clobazam |
| Recommendation |
| When coadministered with stiripentol and clobazam, maximum fenfluramine dose is 0.2 mg/kg twice daily (max 17 mg/day) |
*Clinical discretion is advised when managing ASM regimens.
Download the DIACOMIT Drug Interactions Card
Save this resource for quick reference on documented and potential drug interactions.
Mechanism of Action, Thought To Work in Two Ways:
Direct GABA-A receptor mediation and indirect effects on cytochrome P450
Although the exact mechanism by which DIACOMIT exerts its anticonvulsant effect in humans is unknown, it is thought to:
Increase activity of GABA-A receptors
- Stiripentol may extend the opening time of GABA-A receptor chloride channels, which enhances GABAergic neurotransmission to block an action potential1
- It may also reduce synaptosomal reuptake of GABA and its degradation, leading to higher cerebral GABA concentrations9
Inhibit cytochrome P450 enzymes
- Cytochrome P450 enzymes break down several commonly used antiepileptic drugs, like clobazam. By inhibiting these enzymes, stiripentol may help those drugs to last longer1,10
- In clinical studies, stiripentol increased concentrations of clobazam by about twofold and norclobazam (its active metabolite) by fivefold1
References
1. DIACOMIT® [prescribing information]. Beauvais, France: Biocodex, Inc.; July 2022. 2. DIACOMIT® [summary of product characteristics]. Gentilly, France: Biocodex, Inc.; January 2014. 3. Wheless J, Weatherspoon S. Use of stiripentol in Dravet syndrome: A guide for clinicians. Pediatr Neurol. 2025;162:76-86. 4. Peigne S, Chhun S, Tod M, et al. Population pharmacokinetics of stiripentol in paediatric patients with Dravet syndrome treated with stiripentol, valproate and clobazam combination therapy. Clin Pharmacokinet. 2017;57:739-748. 5. Seiden L, Connor G. The importance of drug titration in the management of patients with epilepsy. Epilepsy Behav. 2022;128(108517). 6. Chiron C, Marchand MC, Tran A, et al; for the STICLO study group. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. Lancet. 2000;356(9242):1638-1642. 7. Morrison G, et al. A phase 1, open-label, pharmacokinetic trial to investigate possible drug-drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clin Pharmacol Drug Dev. 2019;8(8):1009-1031. 8. Fintepla® [prescribing information]. Smyrna, GA: UCB, Inc.; April 2025. 9. Nikels KC, et al. Stiripentol in the management of epilepsy. CNS Drugs. 2017;31:405-416. 10. Fisher J. The effects of stiripentol on GABAA receptors. Epilepsia. 2011;52(2):76-78.