Efficacy
DIACOMIT®(stiripentol) is an effective, FDA-approved antiseizure medication developed specifically for seizures associated with Dravet syndrome in patients 6 months and older (weighing 15 lb or more) and taking clobazam. There are no clinical data to support the use of DIACOMIT as a monotherapy in Dravet syndrome.1
Treat Seizures Sooner
Most children with Dravet syndrome experience their first seizure by 6 months old.49 Now, you don’t have to wait! DIACOMIT is the only treatment specifically for seizures associated with Dravet syndrome in children as young as 6 months. It is indicated for patients 15 pounds or more taking clobazam, and it is proven to significantly reduce generalized clonic or tonic-clonic seizures.1,2
Recommended by Experts
DIACOMIT is recommended as a first- or second-line Dravet-specific treatment, according to an international panel of Dravet syndrome experts.53 When it comes to Dravet syndrome, you don’t have to settle. Place DIACOMIT at the center of your Dravet-specific treatment strategy and offer your patients the chance for profound seizure reduction.
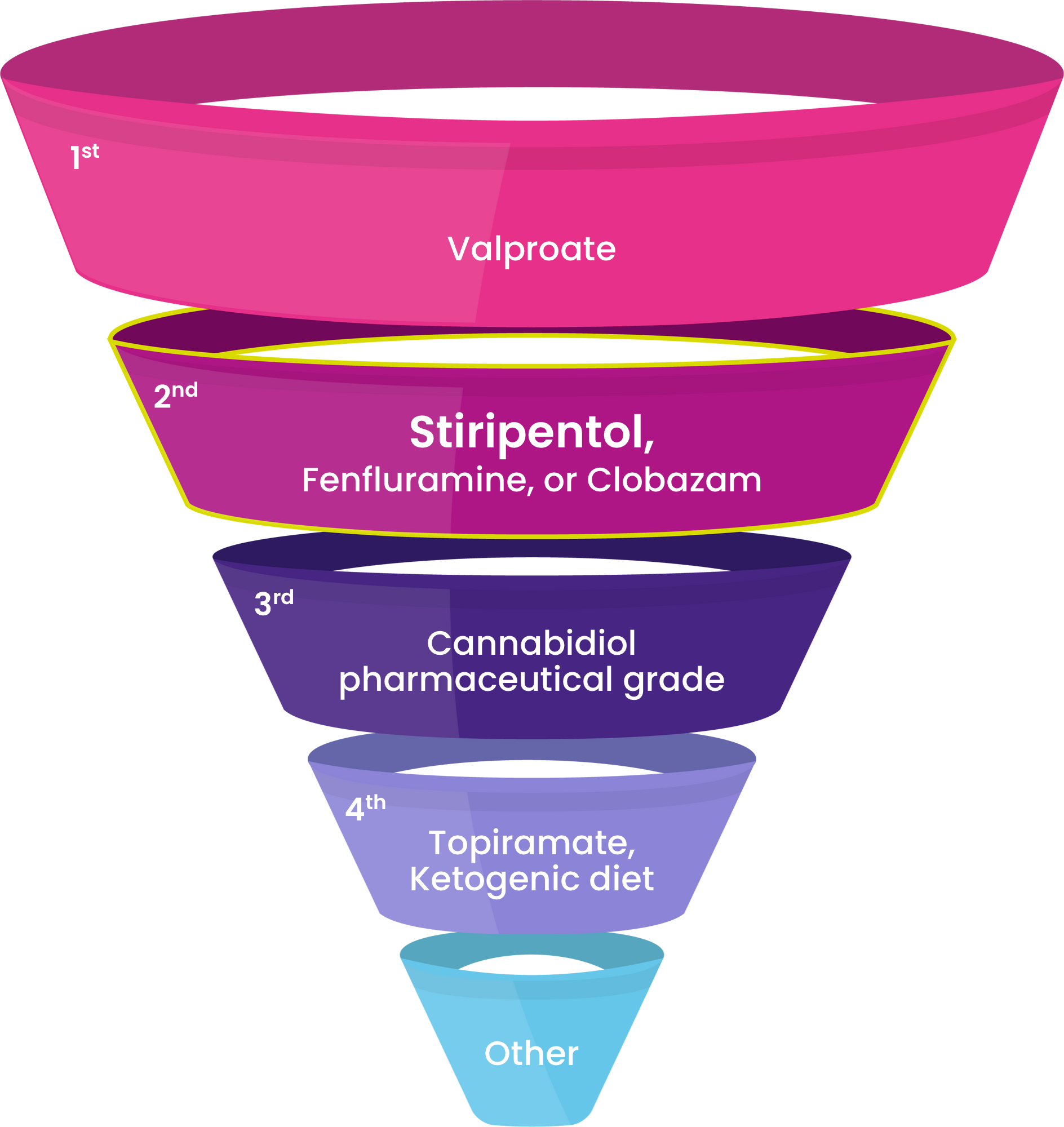
“The demonstrated efficacy of these ‘DS-specific’ medications strongly supports their use earlier in the treatment paradigm…we should redefine our expectations of seizure control, and no longer accept seizures every 1 to 2 months as the best we can do.”53
International Consensus on Diagnosis and Management of Dravet syndrome (2022)
Results with DIACOMIT may vary
Help Your Patients Achieve Profound Seizure Reduction
In two multicenter, randomized, double-blind, placebo-controlled trials, patients with Dravet syndrome on DIACOMIT experienced significantly fewer seizures than those on placebo. After two months on treatment, 58.1% of patients (n=31) saw a 75% or greater reduction in clonic or tonic-clonic seizures with nearly 39% becoming seizure-free.1,2,16 Results may vary.
STICLO Italy and STICLO France Pooled Results Primary Endpoint: Responder Rate
Response was defined as experiencing a greater than 50% decrease in the frequency of generalized clonic or tonic-clonic seizures, and statistical significance was measured with a Fisher Exact Test, generating a p value of p <0.0001.
Additional Endpoints
Median Seizure Frequency
DIACOMIT reduced clonic or tonic-clonic seizures by 84% compared with a 5.8% reduction on placebo after two months on treatment.6
Seizure Freedom
Nearly 39% of patients on DIACOMIT experienced no generalized clonic or tonic-clonic seizures compared with 0% on placebo during the two-month study period.6
STICLO Study Descriptions
STICLO France and STICLO Italy were multicenter, randomized, double-blind, placebo-controlled trials that were conducted according to similar protocols and had comparable results.1,2 Patients were 3 to less than 18 years old; had a confirmed diagnosis of Dravet syndrome; had inadequately controlled seizures on clobazam and valproate; and suffered from four or more generalized clonic or tonic-clonic seizures per month, despite optimized therapy.
Response was defined as experiencing a greater than 50% decrease in the frequency of generalized clonic or tonic-clonic seizures during the second month of the treatment period compared with baseline.2,7 “Seizure-free” was defined as experiencing no generalized clonic or tonic-clonic seizures for the duration of the study.1,6 The baseline was one month on clobazam and valproate before patients entered the double-blind trial for two months on either DIACOMIT (50 mg/kg/day) or placebo.


Review the DIACOMIT Efficacy and Safety Guide
Save DIACOMIT’s proven results for future reference.
More Than Three Decades of Patient Exposure and a Well-Established Safety Profile7, 36, 37, 51
In 2000, DIACOMIT was approved for compassionate use in the United States, and in 2018, it was granted FDA approval.9,10,15 You may recognize DIACOMIT by its generic name, stiripentol, since the treatment was referred to as stiripentol under the compassionate use program until the FDA granted its approval.1,5
DIACOMIT has more than 30 years of real-world use, providing a treatment option specifically for children with Dravet syndrome in 35 countries.40,51 As of 2022, in addition to the United States, DIACOMIT is approved in the European Union, Iceland, Norway, Liechtenstein, Japan, Switzerland, Australia, and Canada.9-15
Why DIACOMIT? An HCP perspective from Dr. Wheless
DIACOMIT reduced seizures for Dr. Wheless’ patient,
allowing him to reduce his patient’s overall medication
burden.
DIACOMIT in the United States
Wirrell et al., 2013: U.S. Retrospective Study (2005-2012)
Researchers collected data from physicians using stiripentol to treat two or more patients with Dravet syndrome between March 2005 and 2012. Thirteen physicians provided data on 82 children. With stiripentol, the majority of patients experienced reduction in frequency of prolonged seizures, rescue medication use, and emergency room or hospital visits.39
In this study, patients on stiripentol and clobazam (n=35) experienced an 80% reduction in seizure frequency.39
Antiepileptic response to treatment was scored on a 5-point scale from marked reduction to marked worsening. Patients on stiripentol, clobazam, and valproate (n=48) experienced a 62% reduction in seizure frequency. Thirty-one patients (n=82) reported adverse events, which were most commonly sedation and reduced appetite.39
| OVERALL SEIZURE FREQUENCY | STP + CLB (n=35) | STP + CLB + VPA (n=48) |
|---|---|---|
| Marked reduction | 11 (31%) | 17 (35%) |
| Mild reduction | 17 (49%) | 13 (27%) |
| No change | 7 (20%) | 16 (33%) |
| Mild worsening | 0 (0%) | 2 (4%) |
| Marked worsening | 0 (0%) | 0 (0%) |
| STP + CLB (n=35) | |
|---|---|
|
Marked reduction |
11 (31%) |
|
Mild reduction |
17 (49%) |
|
No change |
7 (20%) |
|
Mild worsening |
0 (0%) |
|
Marked worsening |
0 (0%) |
| STP + CLB + VPA (n=48) | |
|
Marked reduction |
17 (35%) |
|
Mild reduction |
13 (27%) |
|
No Change |
16 (33%) |
|
Mild worsening |
2 (4%) |
|
Marked worsening |
0 (0%) |
STP = stiripentol; CLB = clobazam; VPA = valproate
Wirrell et al. Study Description
Wirrell et al., 2013 was a retrospective chart review of 82 Dravet syndrome patients across 13 U.S. epilepsy reference centers between March 2005 and 2012. Participating physicians provided data for patients treated with stiripentol anytime in the three years before study onset (median age of initiation: 6.9 years). Data collected included stiripentol dosage, concomitant antiseizure medications, overall seizure frequency, prolonged seizures frequency, rescue medication use, emergency room visits in the year before and during treatment, and adverse effects treatment (reported in 38% of patients; primarily sedation and reduced appetite).39
Study Limitations
The study had limitations including that retrospective chart review is less accurate than prospective counts; patients were on varied other comedications, and their dose changes were not formally assessed; and adverse events were based on chart review, which may have limited reporting of milder side effects.
