DIACOMIT® (stiripentol) is
FIRST and PROVEN
For seizures associated with Dravet syndrome
The FIRST Dravet-specific antiseizure medication:
With nearly 3 decades of real-world experience In over 55 countries1-5
FDA approved for children as young as 6 months6
Weighing 15 lb or more and taking clobazam
A PROVEN, effective treatment that can:
Significantly reduce seizures associated with Dravet syndrome
It is clinically proven to control generalized clonic and tonic-clonic seizures6
Help patients achieve seizure freedom
Nearly 39% of patients were seizure-free during a two-month study period6,7
Proven Results for Your Patients With Dravet
DIACOMIT established efficacy and safety in two double-blind, placebo-controlled pivotal trials with an open-label extension.6,7
DIACOMIT has more than 69,000 patient-years of experience5
Biocodex, a pioneer in Dravet syndrome treatment, develops stiripentol
FDA approves stiripentol for compassionate use in the United States
DIACOMIT is approved in the European Union
Stiripentol is officially FDA approved and now known as DIACOMIT
FDA expands the indication to include patients as young as 6 months
Early Intervention Matters
- Frequent and prolonged seizures can contribute to several medical and developmental issues, including a higher risk for sudden unexpected death in epilepsy (SUDEP)8-12
- Patients with Dravet have a 49% increased risk for SUDEP, which may be associated with tonic-clonic seizure frequency13
Since seizures associated with Dravet may not respond to the standard antiseizure medications (ASMs), it is crucial to determine the right ASM combination for each patient. It is common to treat with multiple ASMs, including others indicated for Dravet.*14
*Clinical discretion is advised when managing ASM regimens
Recommended as a first- or second-line treatment by an international panel of experts14
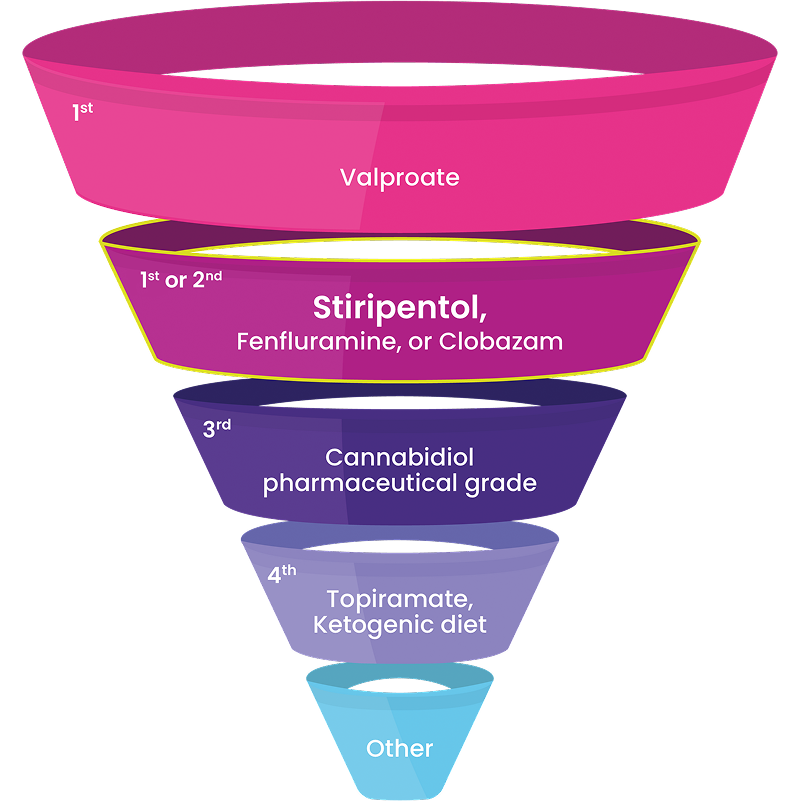
“The demonstrated efficacy of these ‘DS-specific’ medications strongly supports their use earlier in the treatment paradigm… We should redefine our expectations of seizure control, and no longer accept seizures every 1 to 2 months as the best we can do.”
International Consensus on Diagnosis and Management of Dravet Syndrome (2022)
Results with DIACOMIT may vary
References
1. Chiron C, Marchand MC, Tran A, et al; for the STICLO study group. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome dedicated trial. Lancet. 2000;356(9242):1638-1642. 2. Farwell JR, Anderson GD, Kerr BM, Tor JA, Levy RH. Stiripentol in atypical absence seizures in children: an open trial. Epilepsia. 1993 Mar-Apr;34(2):305-11. 3. Bebin M, Bleck TP. New anticonvulsant drugs. Focus on flunarizine, fosphenytoin, midazolam and stiripentol. Drugs. 1994 Aug;48(2):153-71. 4. Perez J, Chiron C, Musial C, Rey E, Blehaut H, d’Athis P, Vincent J, Dulac O. Stiripentol: Efficacy and tolerability in children with epilepsy. Epilepsia. 1999;40(11):1618-1626. 5. Biocodex. Periodic Benefit-Risk Evaluation Report for Stiripentol: Patient Exposure Data, 05 Nov 2023–04 Nov 2024. 19 Dec 2024. 6. DIACOMIT® [prescribing information]. Beauvais, France: Biocodex, Inc.; July 2022. 7. U.S. Food and Drug Administration. CDER Clinical Review. August 2018. https://www.accessdata.fda.gov/drugsatfda_docs nda/2018/206709Orig1s000,207223Orig1s 000MedR.pdf. Accessed May 12, 2020. 8. Wheless JW, Fulton SP, Mudigoudar BD. Dravet syndrome: a review of current management. Pediatr Neurol. 2020;107:28-40. 9. Dravet Syndrome Foundation. What is Dravet syndrome? http://www.dravetfoundation.org/what-is-dravet-syndrome. Accessed November 16, 2020. 10. Wirrell EC, Laux L, Donner E, et al. Optimizing the diagnosis and management of Dravet syndrome: recommendations from a North American consensus panel. Pediatr Neurol. 2017;68:18-24. 11. Sveinsson O, Andersson T, Mattsson P, Carlsson S, Tomson T. Clinical risk factors in SUDEP: A nationwide population-based case-control study. Neurology. 2020;94(4):e419-e429. 12. Zhao H, Long L, Xiao B. Advances in sudden unexpected death in epilepsy. Acta Neurol Scand. 2022;146:716-722. 13. Shmuely S, Sisodiya SM, Gunning WB, Sander JW, Thijs RD. Mortality in Dravet syndrome: A review. Epilepsy Behav. 2016;64(Pt A):69-74. 14. Wirrell EC, Hood V, Knupp KG, Meskis MA, Nabbout R, Scheffer IE, Wilmshurt J, Sullivan J. International consensus on diagnosis and management of Dravet syndrome. Epilepsia. 2022;63(7):1761-1777.